Learning Outcomes
By the end of this lesson, students will be able to:
i. Recognize and explain the striking similarities in chemical and physical properties of elements within the same family, also known as group, in the periodic table.
ii. Analyze how the shared electron configurations of elements within a group lead to their similar behaviors and properties.
iii. Identify and discuss specific examples of element families, such as alkali metals, alkaline earth metals, halogens, and noble gases, highlighting their common characteristics and trends.
iv. Apply the knowledge of element families to predict and explain the chemical behavior of elements in various contexts.
v. Appreciate the significance of understanding element families in organizing, classifying, and understanding the properties of elements.
Introduction
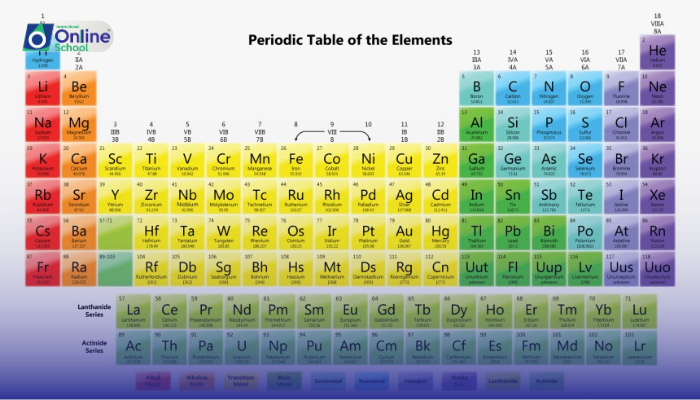
The periodic table, a masterpiece of chemistry, is not merely a collection of isolated elements. It reveals a profound order, a pattern that governs the properties and behavior of elements. This order manifests itself through the existence of element families, groups of elements that share remarkable similarities in their characteristics.
i. Shared Electron Configurations: The Foundation of Similarity
The striking similarities within element families stem from their shared electron configurations, particularly the arrangement of valence electrons. Valence electrons, the outermost electrons involved in chemical bonding, play a crucial role in determining an element's chemical properties.
ii. Alkali Metals: A Family of Highly Reactive Metals
Alkali metals, occupying Group 1 of the periodic table, are characterized by the presence of a single valence electron in their outermost s orbital. This shared electron configuration leads to their remarkably similar properties:
High Reactivity: Alkali metals are highly reactive metals due to their low ionization energies and large atomic radii. They readily lose their valence electron to form ionic compounds.
Similar Bonding Patterns: Alkali metals primarily form ionic bonds with nonmetals, reflecting their tendency to lose electrons.
Predictable Chemical Reactions: The behavior of alkali metals in chemical reactions is highly predictable due to their shared electron configuration and reactivity patterns.
iii. Alkaline Earth Metals: A Step Further in Reactivity
Alkaline earth metals, residing in Group 2 of the periodic table, share similarities with alkali metals but possess two valence electrons in their outermost s orbital. Their properties reflect this shared electron configuration:
Moderate Reactivity: Alkaline earth metals are moderately reactive metals due to their slightly higher ionization energies compared to alkali metals.
Formation of Ionic Compounds: Alkaline earth metals primarily form ionic compounds with nonmetals, similar to alkali metals.
Predictable Chemical Behavior: The chemical behavior of alkaline earth metals is predictable based on their position in the periodic table and their shared electron configuration.
iv. Halogens: Masters of Electron Acquisition
Halogens, inhabiting Group 17 of the periodic table, are nonmetallic elements with seven valence electrons, one less than a full outer shell. Their shared electron configuration leads to their remarkable similarities:
High Electronegativity: Halogens are highly electronegative elements due to their strong tendency to gain electrons.
Diverse Bonding Patterns: Halogens form a variety of bonds, including ionic bonds with metals, covalent bonds with nonmetals, and polar covalent bonds.
Formation of Stable Compounds: Halogens form stable compounds with a wide range of elements due to their strong electron-attracting ability.
v. Noble Gases: A Realm of Stability
Noble gases, nestled in Group 18 of the periodic table, are the most chemically inert elements. With their outer electron shells completely filled, they have no need to gain or lose electrons and possess a unique set of properties:
Exceptional Stability: Noble gases are exceptionally stable due to their filled outer electron shells.
Chemical Inertness: Noble gases are nonreactive due to their lack of motivation to gain or lose electrons.
Unique Physical Properties: Noble gases exhibit unique physical properties, such as low boiling points and lack of color, reflecting their stable electron configurations.
vi. Significance of Understanding Element Families
Understanding element families holds immense significance in chemistry:
Organization and Classification: It provides a framework for organizing and classifying elements based on their similarities, aiding in comprehension.
Prediction of Properties: It allows us to predict and explain trends in element properties, enabling us to anticipate chemical behavior.
Understanding Chemical Reactions: It sheds light on the basis of chemical reactions, enabling us to predict and explain the formation of compounds.
Element families, the remarkable groups of elements within the periodic table, provide a deeper understanding of the similarities and trends in element properties. By delving into the concept of shared electron configurations and group characteristics, we gain valuable insights into the chemical behavior of elements, enabling us to predict and explain their interactions and reactions, further enriching our exploration of the fascinating realm of chemistry.